There are many potential reasons that FMT (Fecal Microbiota Transplant) isn't as effective as hoped. This article investigates these reasons.
This article first appeared on The Power of Poop website.
------------------
Allison Jones is a qualified Nutritionist and Naturopath in Sydney, Australia. Since its inception, many group members in the Facebook FMT Discussion group have tried FMT to treat a diverse range of conditions. For some it has worked, for others it hasn’t. For every success story there is a non-success story, even when the same condition has been treated. In this article, Allison investigates from a naturopathic and functional medicine viewpoint the many reasons why FMT may not always work.
The contents of this article should not to be construed as medical advice. Different situations call for different treatment plans. Always discuss your options with your healthcare provider.
Fecal microbiota transplant (FMT) has captured the attention of researchers, health practitioners and those with chronic illness like almost no other treatment intervention in recent medical history, probably due in part to its rapid and high success rate for antibiotic-resistant Clostridium difficile (C. diff) infections but also due to its novel nature. There is no doubt that FMT can be lifesaving in cases of C. diff infection and there has been some discussion that it should be used as a first line intervention for C. diff before antibiotics because it is so effective, so quickly.
In the US, FMT is only permitted to treat C. diff that does not respond to multiple courses of antibiotics. However, in other countries it is increasingly being used experimentally to treat other digestive conditions and complex multi-system conditions that often do not present with overt digestive disturbances, such as depression, anxiety, chronic fatigue syndrome and immune deficiency. There is currently no established evidence for the use of fecal transplants in conditions other than c. difficile infection, however the demand for this treatment is very high, no doubt driven by the attention the gut microbiome has received in recent years and the desire for sustained resolution that those with chronic illness seek. FMT is performed in licenced medical clinics or at home under guidance of a health practitioner. Some patients choose to do FMT independently without supervision.
Given the incredible attention fecal transplants have received, the high cost to undertake this treatment at a medical clinic and the ongoing patient-driven demand, clinical success rates should be assessed. Some who have received fecal transplants in a medical clinic for non- C. diff conditions have reported failure of the treatment, even when the strict instructions provided by the clinic were followed to the letter including pre-treatment antibiotics, pre-treatment diet and post-treatment diet. Many patients measure success not only by symptom improvement, but by using before and after stool testing offered by a range of laboratories.
Patient feedback indicates that clinics do not conduct long term follow up i.e. contact patients at the 6 months, 12 month and 2 year mark to ascertain how they have travelled since FMT. Given the experimental nature of the procedure, this is a concern. There also appears to be a clinical culture of focusing on successes and dismissing non-successes and ongoing side effects. This is disappointing as there is more to be learned from failure than success and valuable information is being lost.
Possible reasons for failure of FMT are discussed below with a view to educating patients and encouraging clinics to collect and report data with a view to promote improvements in treatment protocols. Such a database would give scientists a starting point to identify trends, form hypotheses and set up research programmes to test them.
Reason 1: Insufficient infusions
There is an established protocol for treatment of C. diff that assumes the infection can be cleared with as little as 2-3 infusions[1]. However, for other conditions there is as yet no established protocol. Some patients are advised they may need 10 treatments, others anywhere up to 50 treatments. Ulcerative Colitis patients in our discussion group occasionally experience remission from short term FMT but many more report that multiple treatments over many months are required to send their condition into remission.
Patient tip: Do not expect miracles. If using a clinic, consider testing a donor beforehand in case you need to continue FMT at home.
Reason 2: Not being able to retain the infusions for long enough
Some patients find the infusions difficult to retain for a variety of reasons. “The longer the better” seems to be the motto when receiving fecal transplants as this gives the microbiota in the donor stool more time to adhere to the gut wall where they can settle in and proliferate.
Patient tip: Less is more if you struggle to retain the infusion. Try small infusions over the space of an hour. Lie rear ended elevated or upside down if you are athletic. Doing the infusion before bed works for some, provided your donation arrives at that time. Drugs like Lomotil and Immodium slow movement of the gut and can also be helpful. Foods such as bananas and rice (white rice is often easier to digest) are also good binding foods. Lastly, tannin-containing foods are astringent, helping to minimise the risk of loose stool – good examples are blueberries and green tea.
Reason 3: Incorrect diet
Clinics that perform fecal transplants generally advise to follow a low fibre diet before treatment and high fibre diet after treatment. High fibre is required post-treatment to ensure the donor microbiota have sufficient food (substrate) to proliferate.
This dietary advice would benefit from being extended to specify a broader range of prebiotic foods to maximise diversity of gut microbiota in a healthy colon. There are many prebiotic foods readily available, so it makes sense to provide a broad list and advise patients to eat as widely as possible from it, without overdoing it, especially where IBS has been an issue as excess fibrous foods often worsen IBS.
Patient Tip: Below is a list of different prebiotic foods, ensure to choose those that are appropriate for your situation (eg. no inulin for those with IBS) and try to eat as wide a variety as possible! Exercise caution when introducing a new food, try to avoid large servings of these foods initially. There are many more prebiotic foods available than this list which is a starting point.
- Cooked and cooled white potato and white rice for resistant starch
- Tigernuts (resistant starch)
- Raw green banana flour (resistant starch)
- Green-tipped bananas, green plantains (resistant starch)
- Onions, leeks, garlic (inulin)
- Slippery elm
- Flaxseed/Linseed (freshly ground is best and stored in a refrigerator)
- Gluten-free oats, oat bran and buckwheat
- Legumes – take care to prepare these adequately
- Psyllium
- Raw nuts
- Broccoli, asparagus, brussel sprouts, cabbage
- Avocado
- Cacao
- Prebiotic supplements: Inulin, acacia fibre, PHGG (eg. SunFiber), glucomannan, Bimuno, lactulose, larch arabinogalactans, D-Mannose
Additionally, patients should be advised to avoid known inflammatory foods that may trigger inflammation and affect success of the transplants. The new donor microbiota needs a healthy environment in which to prosper – an inflamed one is not that. Foods such as high Omega 6 vegetable/seed oils, excess sugar, alcohol, gluten grains, soy, dairy and corn are known to irritate the gut lining and contribute to inflammation and an inappropriate immune response. Some may tolerate high quality dairy, but it’s important to be cautious. In some conditions such as IBD and ankylosing spondilitis, starches may need to be minimised or avoided. A high fat and high protein diet may also displace prebiotic foods and negatively influence the diversity and makeup of the microbiome so should also be avoided where possible[2] (also, see Reason 6).
Patients with specific conditions may also benefit from adherence to additional guidelines such as low FODMAPS (for those with IBS), SCD (Specific Carbohydrate Diet) for Crohn’s and colitis, or the Paleo autoimmune approach for those with autoimmune disease. Of course, some of these ideas are relatively new and not everyone will agree on their merit or which approach is best. A decision of which diet to use and when to make changes should be made between health practitioner and patient.
Patient tip: Always consider your own personal situation when making a decision about diet. Work with your qualified health practitioner to design a diet suited to your individual needs.
Reason 4: Other factors in the digestive environment that are not appropriately addressed
The large intestine is not an isolated organ in the body. Fecal transplants, while amazing in many cases, are rarely a magic bullet for those with chronic conditions. They are just one part of the healing process. Other areas of the digestive system should be investigated and addressed as part of the fecal transplant protocol.
Hypochlorhydria, or low stomach acid can be caused or exacerbated by a few factors: thyroid conditions, older age or nutrient deficiencies such as thiamine and zinc[3]. Low stomach acid can result in two problems that may affect the success of fecal transplants. Firstly, it can allow certain microbiota or pathogens to overgrow and re-populate the large (and small) intestine, a consideration both before and after FMT[4]. Secondly, in the absence of adequate stomach acid, animal proteins are poorly digested and may putrefy or ferment instead of being adequately digested and absorbed in the small intestine. In a practitioner-only presentation given by Bioceuticals in Australia, clinician and researcher Michael Ash stated his belief that adequate stomach acid is required for commensal bacteria to adhere to the lining of the digestive tract and to reproduce.
Lack of pancreatic enzyme production can also be a factor here. This putrefaction dysbiosis poses its own risks, with suggestions that it can promote the growth of colon cancer if not appropriately addressed[5]. It will also reduce the likelihood of the new donor microbiota thriving and colonising.
Pancreatic enzymes have a dual role in microbiome health:
- Pancreatic enzymes degrade bacteria and their toxins such as cholera. They also destroy the outer layer of species of Escherichia coli, Klebsiella pneumoniae, and
- Pancreatic enzymes break food down in to smaller and smaller particles for absorption in the small intestine. When the enzymes are not produced adequately, undigested food particles (especially proteins) may cross the intestinal wall especially when the integrity of the epithelium is compromised. As the immune system of the gut is accustomed to very particular protein sizes, this may trigger an immune response including a pro-inflammatory state.[6] See Reason 5 for more information.
Address all possibilities well before (and after) a fecal transplant. Consider comprehensive digestive testing such as the Genova GI Effects which can identify bacteria, fungal and parasitic infections and also pancreatic enzyme status (via pancreatic elastase measurement). Observing stool after it has been passed may also be helpful in determining enzyme and bile sufficiency – smelly stool, floating stool, a greasy appearance and/or pale stool are all indications that either the stomach acid, pancreatic function or bile production and flow is not sufficient. Pancreatic function can be supported by digestive enzymes and bile can be supported by consuming adequate cholesterol-containing foods, along with taurine and glycine. Hydrochloric acid can be supported by optimising thyroid function, eating in a relaxed way, improving nutrient status or with supplements such as Betaine HCl or bitters. Specific herbs are also very helpful in restoring bile flow – always consult an appropriately qualified practitioner, especially where herbs are concerned. Adequate stomach acid is also essential for the right signalling to occur for the release of pancreatic enzymes and bile.
To summarise the points above, all digestive secretions – hydrochloric acid, pancreatic enzymes and bile must be produced in adequate amounts in order to maintain a healthy balance of bacteria as these are all bactericidal and part of the complex management system of the digestive tract[7].In Australia, Bioscreen testing (and equivalent) is useful when conditions such as CFS, ASD, ADHD, anxiety, OCD or depression are present, however this will only identify bacteria and parasites. GI Effects testing is much broader but doesn’t look at the same set of microbes as selected by Bioscreen for their analysis.
Patient tip: Fecal transplants are only one tool in the digestive illness toolbox. Make sure you have investigated other issues in the digestive system that could be contributing to dysbiosis before going to the expense of FMT as these factors may still be present after FMT.
Reason 5: Not addressing the entire individual and the state of the immune system
This point follows on from Reasons 3 and 4 and is substantial and broad. Most of the body’s immune function is located in the digestive tract, comprised of commensal bacteria, the gut-associated lymphoid tissue (GALT), tight junctions forming the barrier function of the epithelial layer and immune factors such as Secretory IgA (SIgA), produced at mucosal surfaces such as that of the digestive tract. Inappropriate and erratic inflammatory and immune processes that are not addressed before fecal transplants can lower the chances of success. New commensal bacteria are not likely to thrive and proliferate in a digestive environment that is in a state of disarray and inflamed. The images below are very helpful when trying to visualise the environment in the digestive tract.
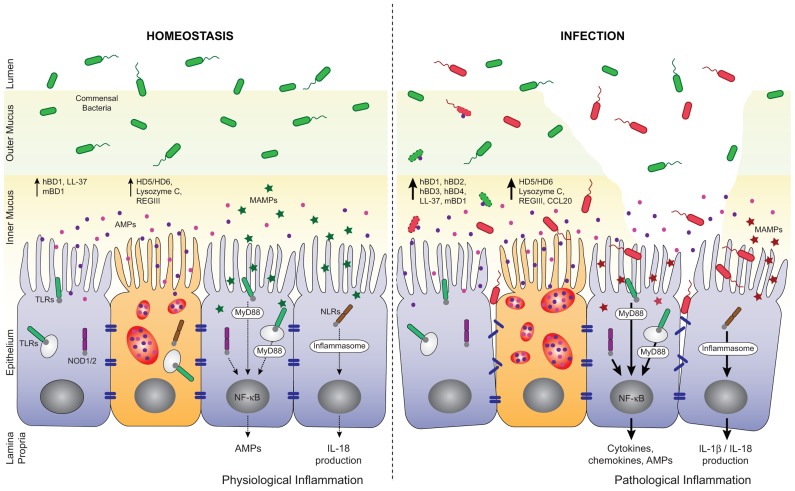
regulation of AMP secretion by TLRs and NLRs during homeostasis and infection – http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3466489/figure/F2/
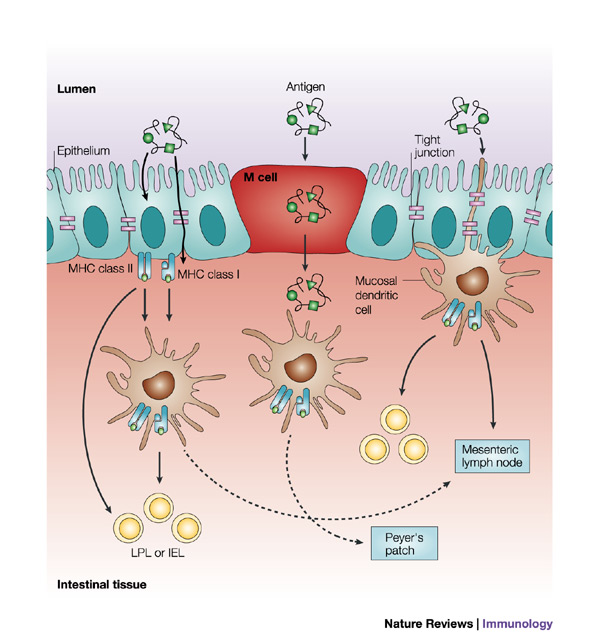
Gut-associated lymphoid tissue (GALT) is a type of mucosa-associated lymphoid tissue (MALT) which are situated in various parts of the body as the immune system’s first line of defense. Examples of these tissues include the adenoids, tonsils and the Peyer’s patches found in the ileum (the furthest part of the small intestine) which store immune cells such as T and B lymphocytes that defend us from pathogens. Peyer’s patches surveil the environment for threats using M cells to “sample” the environment, presenting microbial and dietary antigens for either a pro-inflammatory or tolerogenic immune response[8].
Tight junctions are part of the junctional complex system that forms the epithelial barrier in the digestive tract. For various reasons, this barrier function may be compromised, leading to increased intestinal permeability (“leaky gut”) which sets the scene for increased inflammation and inappropriate immune responses if the conditions are right (genetics and diet for example). This is because a higher number of antigens are able to pass the barrier where they then meet the immune system which may respond in a number of ways, either positively or negatively[9].
Secretory IgA (SIgA) is the most populous type of antibody in the intestinal region. SIgA has a highly complex role in regulating the immune system, maintaining “tolerance” (that is, preventing the immune system from attacking the host itself by balancing Th1 and Th2 activity) and clearing antigens and pathogens from the intestinal lumen with highly co-ordinated activities. SIgA also prevents many pathogenic bacteria from releasing virulence factors, adhering to the intestinal epithelium and forming biofilm[10]. Related to Point 6 below, researchers believe that adequate levels of SIgA ensure there is sufficient biofilm for commensal bacteria[11] [12].
The immune disarray generally occurs due to a combination of diet (inflammatory and lacking in prebiotic foods), genetics, chronic stress (this down regulates production of SIgA causing an upregulation of pro-inflammatory cytokines[13]), increased intestinal permeability, poor sleep, inheritance of poor microbial diversity and other immune disturbances. It may contribute significantly to chronic conditions involving different body systems such as brain inflammation (implicated in depression for example[14]), different types of arthritis[15], autoimmune diseases and autism[16] [17] [18].
Increased intestinal permeability (leaky gut) also needs to be addressed to restore correct functioning of the immune system. Of course, some of these things are often difficult to address before fecal transplants but it pays to address them as much as possible to protect your investment in fecal transplants. For those with IBD, being in the middle of a flare can also affect the success of fecal transplants, so it’s important to work with your medical practitioner to subdue this prior to FMT.
Genova’s GI Effects measures SIgA, other labs will also be able to measure this. If the level of SIgA is too high, it indicates an inflammatory response is currently active and the SIgA may be produced at inadequate levels at a later date (suggest re-testing in 3 months to check on it again). If the SIgA is too low, address stress and consider the Biocodex strain of Saccharomyces boulardii which has been shown to restore levels of SIgA. It needs to be taken for a minimum of 6 months and the level should be re-tested.
Address all other factors listed above such as stress, diet and sleep. Key nutrients to be aware of for optimal immune function include selenium, Vitamin A (not betacarotene), Vitamin D, zinc and iron. Caution is recommended with iron as it can be used to build biofilm – if levels are low, it must be taken with lactoferrin to prevent its use for biofilm formation[19]. Lactoferrin is also believed to assist in the absorption of iron in the small intestine in both infants and adults, on account of the lactoferrin receptors found along the small intestine[20].
Patient tip: Some general factors to consider along with diet – chronic stress levels (and the perception and response to stress), use of targeted and low-risk supplements or medications to help the body dampen down inflammation (curcumin for example), spending quality time in nature to take advantage of its healing effects[21], quality sleep.
Reason 6: Ongoing presence of biofilm infections or standard infections
If you’ve never heard of biofilm, you’re not alone. A widespread assumption in both the lay and medical community is that pathogenic bacteria are just floating about, ready to be killed off with antimicrobial medicines or herbs. This is not the usual pattern of chronic microbial infections and leads to quite a significant potential reason for fecal transplants often failing. All organisms, from tiny microbes, to plants, to great white sharks, have protective mechanisms in place to allow them to live. When these organisms are in challenging environments, they develop quite sophisticated survival mechanisms as they face each new challenge. Biofilm is one such survival mechanism used by microbes and it is everywhere around us from dental plaque to slimy rocks and surgical instruments. The biofilm protects the microbes from host immune defenses like an “invisibility cloak”.
Microbiologists who study biofilms believe that up to 80% of chronic infections are biofilm infections rather than planktonic infections, where bacteria are more easily accessed by antibiotics and other antimicrobials[22]. In humans, biofilm is implicated in chronic ENT and gut infections (including SIBO and large intestine dysbiosis), tooth decay, and potentially many more illnesses,and it can house both bacteria and fungi. With this in mind, it is important to note that not all biofilm is bad – commensal bacteria (those that have a symbiotic, beneficial, relationship with their host) also use biofilm to communicate with each other and reproduce. Unfortunately, biofilm itself can’t be measured, however in the presence of chronic infections, it should be assumed that biofilm is present and an important factor to be addressed.
This means that a supervised, intensive biofilm protocol is best carried out well before fecal transplants (and for an appropriate length of time) in order to clear out dysbiotic and pathogenic bacteria properly. This reduces the chances of these bacteria remaining in the biofilm, safe from antibiotics and becoming more resistant to antimicrobials. Reducing the levels of dysbiotic bacteria and fungi means that new incoming bacteria from donor stool has a better chance of adhering to the gut wall (since bacteria themselves produce their own antibiotics that may kill new bacteria). A biofilm protocol will include appropriate testing to assess which specific dysbiotic bacterial species are present and also if there are any fungi or yeast that are present at high levels. In severe cases, biofilm protocols may need to be followed for between 9-24 months. The biofilm aspect must be taken seriously if one is to succeed with fecal transplants. A good biofilm protocol will ensure that the biofilm for commensal bacteria is nourished along with the removal of biofilm containing dysbiotic and pathogenic bacteria.
Here are some documents summarising biofilm as it relates to human health:
Developmental_regulation_of_microbial_biofilms
Presentation from Dr. Anju Usman who pioneered biofilm treatment (from page 10)
Persistent infections, symptomatic and asymptomatic
Prior to fecal transplants, many clinics recommend a (sometimes very long and intense) antibiotic protocol to clear out existing dysbiotic or pathogenic microbes. The questions to ask here are: what are the potential side effects of the antibiotics (eg. potential neurological and other side effects such as with fluroquinolones[23], mitochondrial damage[24]), are there safer antibiotics that can be used, can targeted (and not too indiscriminate) herbal antimicrobials be trialled first, does the duration of the antibiotic program need to be so long, what sort of before and after testing of stool is done to ensure the antibiotics are worthwhile, is biofilm addressed and are the biofilm medications or supplements safe? Please remember that antibiotics are lifesaving in many situations, so consult with your health practitioner appropriately when deciding whether or not to use antibiotics.
Patient Tip: Remember, do your own research and always work with an appropriately qualified healthcare practitioner rather than embarking on a biofilm protocol on your own. There are some potential pitfalls – some biofilm enzyme products (biofilm disruptors) contain EDTA which can bind to and excrete essential minerals such as zinc (essential for immune function along with 200+ enzymatic reactions). Also, overuse of NAC may affect mucosal health negatively as it is a mucolytic.
Reason 7: Donor stool is of poor quality
A common assumption made by patients seeking fecal transplants from a medical clinic is that donor stool will be of very high quality. Patients report that this is not always the case. Few clinics test their donors for microbial diversity prior to donation, claiming that there is no universally recognised measure for this and there is a wide range of ‘normal’. With demand for donor stool possibly outstripping supply, dysbiosis may continue to be a problem.
Donor testing by clinics focuses on elimination of fecal pathogens (eg. parasites and H. pylori) and blood borne communicable illness (eg. hepatitis and HIV). This rules out obvious risks and limits the legal liability of the clinic. However even with the absence of major pathogens, using stool that is lacking in microbial diversity to treat a patient with existing dysbiosis is not smart medicine. Donors may seemingly be in good health, but they may harbour an overgrowth of the usual bacteria found in humans or an undergrowth of Bifidobacterium for example. All donors should have thorough testing for dysbiosis along with the standard screening, especially considering that the treatment is prohibitively expensive and still in the process of gaining awareness and acceptance. The fact that experts don’t agree on an appropriate test does not justify not doing any testing. For fecal transplants to gain widespread acceptance, all risks must be addressed. Failing to test for dysbiosis does not help those with chronic illness and risks the reputation of FMT amongst patients and the medical community.
Patient tip: Ask questions if you are seeking fecal transplants from a medical clinic. Do they test donors for dysbiosis (overgrowth of normal gut microbiota) or just known pathogens? If they do not test for dysbiosis, ask why not. FMT at a clinic is expensive and the risk of picking up dysbiotic or pathogenic microbe is real. There have been reports of new dysbiotic bacteria being detected on stool tests for the recipient following transplants, where that bacteria has never appeared previously on repeated stool testing conducted over a period of two to three years prior to the fecal transplant treatment. Also, ask your clinic about the health of the donors – are they of healthy weight, good mental health, in good general health. Don’t be afraid to ask – it is extremely important.
Reason 8: Donor stool bacteria not thriving
As already detailed, it is ideal for the health of the overall digestive environment to be addressed long before fecal transplants are conducted, so as to optimise the chances of success in getting the new commensal bacteria to reproduce and colonise successfully.
SIgA is quite significant in the ability of commensal bacteria to adhere and proliferate – it coats the bacteria and in newborns, this directs the immune system to accept the commensal bacteria as they are then viewed as symbiotic by the immune system[25].
Not enough secretory IgA will affect the ability of the microbiota to adhere to the gut lining and colonise as it is believed that SIgA promotes a symbiotic relationship between host and commensal bacteria by reducing intestinal pro-inflammatory responses against commensal bacteria[26].
If existing pathogenic or overgrown bacteria are not addressed prior to a fecal transplant, the antibiotic substances produced by these bacteria may pose a significant challenge to the establishment of a healthier microbiome when a fecal transplant is performed. New incoming bacteria must have enough space on the epithelium (gut wall) to establish themselves – this is very hard to do if the space is occupied by pathogens or overgrown bacterial species.
Another factor to consider here is the mucosa and epithelial layer of the gut and whether these are healthy or not. Apart from minimising inflammation, glucose from starchy foods is essential for production of mucin, the primary molecule in mucus. The mucosa is a protective barrier that allows SIgA to exert its effects on pathogens. Glycoslyation of proteins is essential for integrity of the tight junctions of the intestine (preventing increased intestinal permeability) and therefore preventing the entry of pathogens and a deleterious immune response. Sufficient glucose is also needed to produce the proteoglycans and non-proteoglycan polysaccharides for the extracellular matrix (ECM), which provides structural integrity to barriers such as skin and the digestive tract[27]. Collagen is also important structurally for the ECM. Specific amino acids and other nutrients are needed for collagen production – these can be found in generous amounts in gelatinous foods and quality whole-protein foods. Sufficient copper and Vitamin C levels are also a requirement for collagen production.
Further reading about the importance of dietary glucose.
Patient tip: Eat plenty of gluten-free starchy foods such as potatoes, sweet potatoes, yams, yuca/cassava, taro, plantains, white rice. Include gelatinous bone broth and stews in the diet to promote collagen production and eat high Vitamin C foods such as red capsicums (bell peppers), strawberries, kiwi fruit and citrus. Copper foods such as chocolate, avocado and beef liver are also useful (the first two also contain prebiotic fibre) – exercise caution in the presence of a zinc:copper imbalance.If SIBO or other conditions such as ankylosing spondylitis is a concern, dextrose (glucose) may be an option for providing glucose if starchy foods are restricted. Otherwise, foods with a low fermentation potential, eg. jasmine rice, may be tolerated.
Reason 9: Hidden reservoirs in the body re-infecting the small and large intestines
This point is perhaps most relevant for those dealing with Streptococcus spp. infections, including those diagnosed with PANDAS[28] or those dealing with the negative effects of Streptococcus spp. overgrowth in the large intestine (which may not necessarily be PANDAS as such). Streptoccocus species are many and varied and many tend to colonise in the sinuses and the oropharynx region including the oral cavity, tonsils and adenoids. If these areas of colonisation are not addressed and the conditions are right (low digestive secretions for example and lack of lactobacilli and bifidobacterium), the streptococcus is allowed to travel to the large intestine where these species may proliferate and create symptoms.
Patient tip: Investigate this potential issue and address it appropriately with medical guidance. The good news is that many practitioners believe that biofilm enzymes taken orally have systemic effects, so they may help eradicate biofilm in the oropharynx region for example. In severe cases of PANDAS, some patients have benefited greatly from removal of the tonsils and adenoids, yet others have not[29]. Of course, if that can be avoided, it is ideal and should only be considered as a last resort in conjunction with medical advice.
Conclusion
Fecal transplants have a good track record as a treatment for antibiotic-resistant C. diff but are less established for all other conditions, so are considered experimental. Most clinics do screening of donor stool, but this screening may not be enough. There are many ways in which you can increase the chances of success with fecal transplants. The purpose of this article is not to put fear or further hurdles in the path of the chronically ill, but to provide honest, constructive information to increase the chances of successful treatment.
Looking for help? Get in touch...
I regularly work with people who want to investigate aspects of their digestive health before getting FMT and also other clients who want post-FMT dietary advice.
I've observed that many people who are getting FMT need more investigations before having it done. The FMT process is expensive, so it makes sense to aim for the best results possible. For example, many people having FMT actually have SIBO which has been undiagnosed. While there is some emerging research that FMT may be helpful for SIBO, this is not established.
You can either book a Clarity Call (no treatment advice) or you can work with me on an ongoing basis by booking the Initial Consultation. Book here: Work with me
References
[1] http://www.nejm.org/doi/full/10.1056/NEJMoa1205037
[2] http://humanfoodproject.com/can-a-high-fat-paleo-diet-cause-obesity-and-diabetes/
[3] http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3312726/
[4] http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3038963/
[5] http://www.ei-resource.org/illness-information/environmental-illnesses/candida-and-gut-dysbiosis/
[6] Karpa, K. (2003). Bacteria for breakfast: probiotics for good health. Victoria, B.C: Trafford.
[7] http://www.townsendletter.com/FebMarch2015/sibo0215.html
[8] http://www.mdpi.com/2072-6643/5/3/771/htm#fig_body_display_nutrients-05-00771-f001
[9] http://www.mdpi.com/2072-6643/5/3/771/htm#fig_body_display_nutrients-05-00771-f001
[10] http://www.nature.com/mi/journal/v4/n6/full/mi201141a.html
[11] http://iai.asm.org/content/72/4/1929.full
[12] http://www.nature.com/mi/journal/v4/n6/full/mi201141a.html
[13] http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3845795/
[15] http://www.theatlantic.com/health/archive/2015/01/joint-pain-from-the-gut/383772/
[16] http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3564498/
[17] http://www.nature.com/ncomms/2014/141210/ncomms6748/full/ncomms6748.html#close
[18] http://journal.frontiersin.org/article/10.3389/fendo.2014.00029/abstract
[19] http://www.jleukbio.org/content/81/2/393.full
[20] http://www.ncbi.nlm.nih.gov/pubmed/16261254
[21] http://heapro.oxfordjournals.org/content/18/3/173.full
[22] http://www.ncbi.nlm.nih.gov/pubmed/23025745
[23] http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3981197/
[24] http://www.ncbi.nlm.nih.gov/pubmed/23825301
[25] http://www.nature.com/mi/journal/v4/n6/full/mi201141a.html
[26] http://femsim.oxfordjournals.org/content/56/2/185
[27] http://www.ncbi.nlm.nih.gov/books/NBK26810/
[28] http://www.nimh.nih.gov/health/publications/pandas/index.shtml/


Wow. This is one of the best articles I have read about FMT. I assumed you were an integrative medical doctor.
Thanks Suzanne! I hope it helped you. I spent a lot of time writing it, which I think was worthwhile.